RNAi Biopesticides
Partner with Demeetra to Combine Gene Editing Technologies for Cost-Effective Scalable RNAi Biopesticides
Recent publication selected as MDPI Editor’s Choice Article1
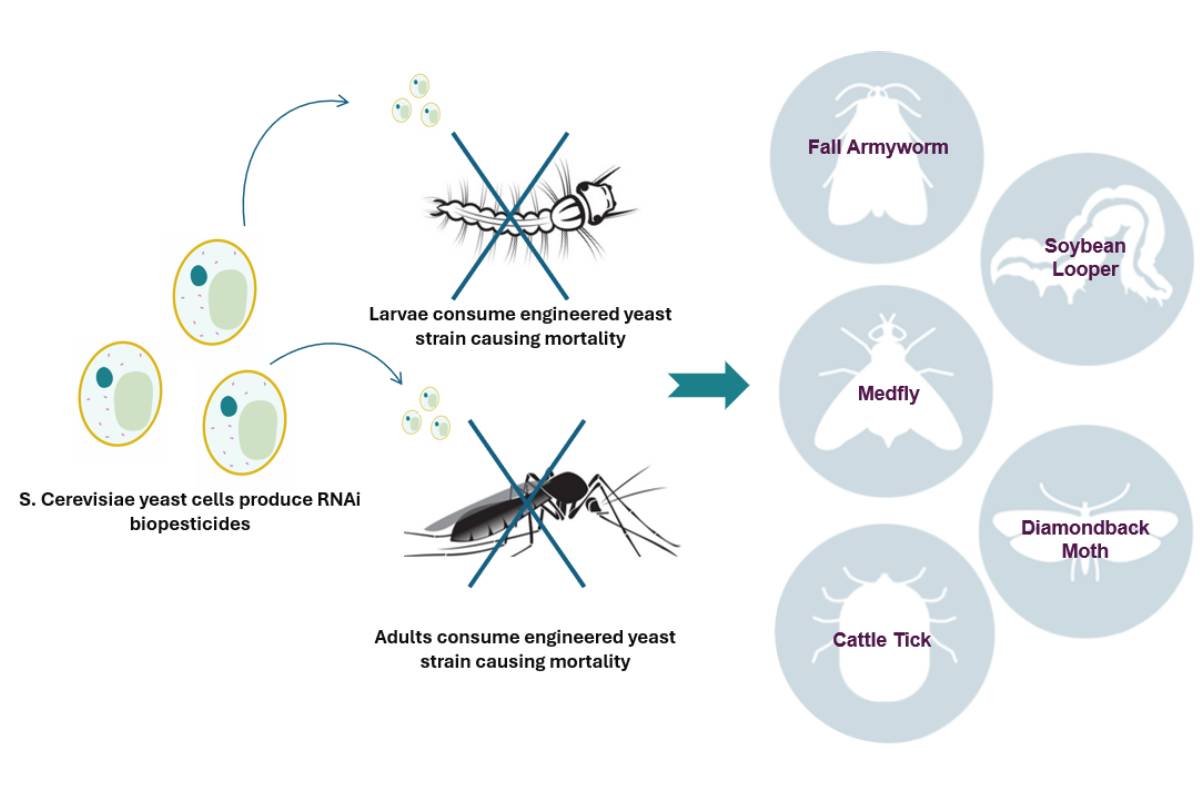
Demeetra’s biopesticide platform can be applied to agricultural or environmental pest. Interfering RNA (RNAi) offers an effective, precise, and eco-friendly alternative to chemical insect pest management. However, significant limiting factors include the high cost of developing GMO crops to express RNAi as well as chemical synthesis at industrial scale. Engineered S. cerevisiae (yeast) as a bioprocessing platform potentially resolves both the cost and environmental stability hurdles associated with RNAi.
Here we describe the advanced gene editing technologies and processes Demeetra scientists employ to rapidly generate stably expressed RNAi biopesticides. The platform’s performance proved highly effective in semi-field trials. The first target was mosquitoes, but this proprietary combination of technologies and methods can be replicated for any pest that can consume yeast.
The Technologies and Processes
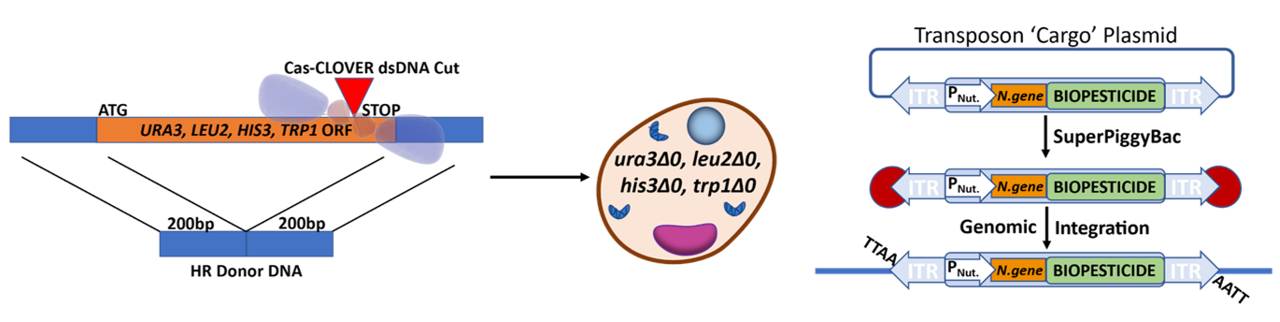
Utilizing Cas-CLOVER and piggyBac transposase together: Left- Cas-CLOVER seamlessly eliminates nutritional genes essential for yeast to grow. Middle: yeast maintained on media supplemented with essential nutrients prior to transformation. Right: piggyBac transposase (red enzyme) recognizes transposon target sequences called inverted terminal repeats (ITR) and integrates RNAi biopesticide transgene along with nutritional gene (PNut = nutritional promoter, N.gene = nutrition gene) for selection of stable clones.
First, the Cas-CLOVER targeted nuclease system, a more precise alternative to CRISPR Cas92, was used to knockout essential nutritional genes that were subsequently rescued by the RNAi transgenic vector for selection of highly expressing stable clones. To do this we completely deleted the open reading frames (ORFs) of auxotrophic genes, ura3, leu2, trp1, and his3. Having multi-auxotrophic selection options in the engineered yeast resulted in a versatile bioprocessing platform. Strains can be rescued on media lacking the accompanying supplementation through multi-vector stable transgenic introduction of nutritional marker genes delivered by the piggyBac transposase, which also drives the expression of the RNAi biopesticide.
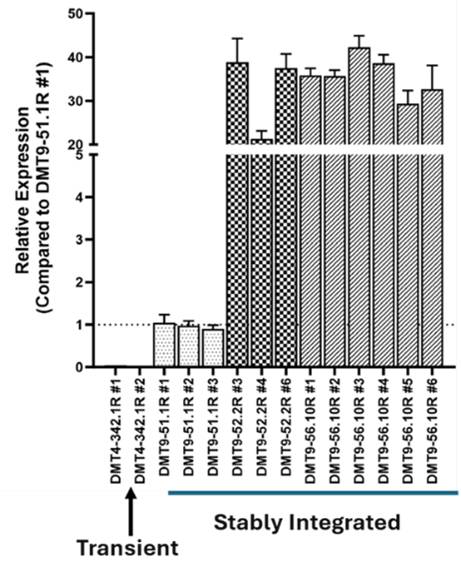
PiggyBac transposase semi-randomly integrates transposons at ‘TTAA’ genomic sites, favoring non-coding open chromatin. This results in stable and high expression of the gene of interest. Using this transposase-based multi-auxotrophic selection platform enabled detection of individual cell clones with a dynamic range of expression levels, including over 350-fold increases from transient expression.
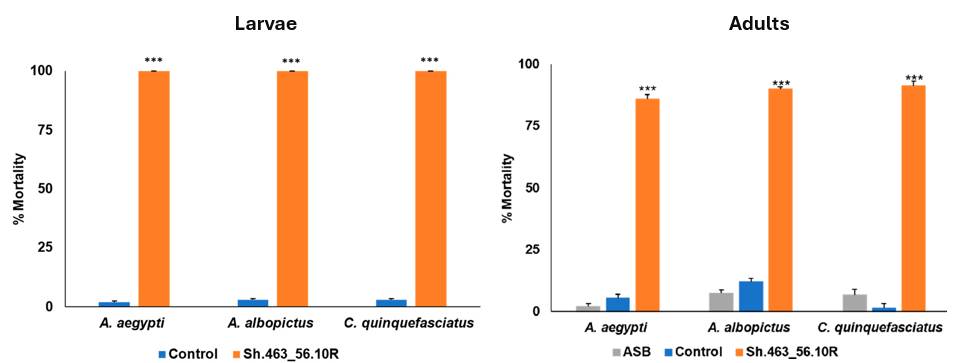
Selected clones performed extremely well, achieving nearly 100% mortality in third-party laboratory and semi-field trials (shown below) for both larvae and adult mosquito vector species for Malaria, West Nile, and Zika. At the same time, non-target species were shown to be unaffected, with water fleas, planktonic crustaceans, beetles, and flies maintaining 100% survival in the lab.
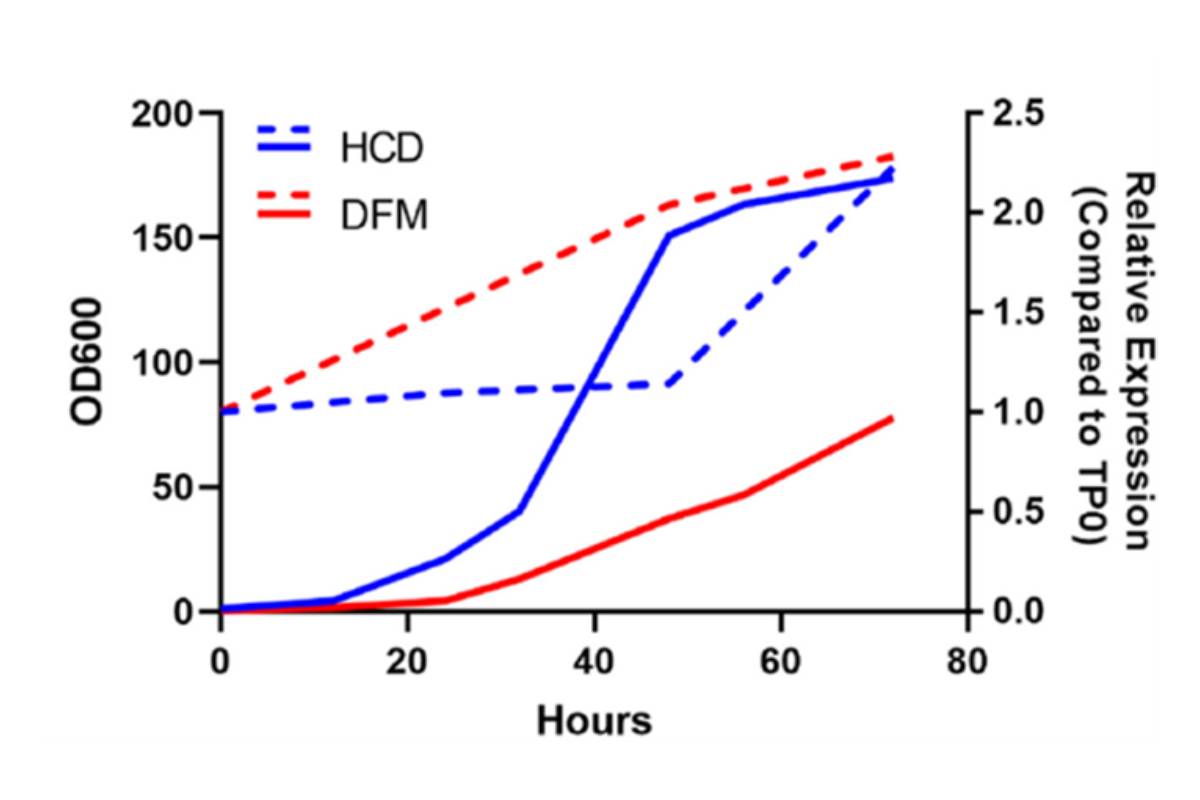
Scalable Production through Fermentation
A significant advantage of this platform is its scalability. Pilot industrial fermentation runs confirmed robust yeast growth and stable expression of the RNAi. These fermentations can be scaled to industrial levels, producing kilogram-scale quantities of dried yeast biopesticide. Heat killed and dried yeast can be stored at room temperature where the RNAi is locked in the yeast cells and remains active. Dried yeast can be differentially formulated for a diversity of pest management strategies. This scalability is crucial for the global deployment of this technology, making it accessible and affordable for agricultural applications.
Conclusion
Recently in the Journal of Fungi4 and Fermentation1, Demeetra scientists demonstrated applying targeted gene knockouts with Cas-CLOVER followed by stable RNAi strain development with piggyBac transposase. This innovative approach not only offers targeted pest control but also ensures scalability and cost-effectiveness, paving the way for its widespread adoption.
Partnering with Demeetra is more than access to technology licenses. At our internal labs in Lexington, KY we optimize gene editing technologies in commercially applicable systems including mammalian, microbial, and plants. Our team of peer-reviewed and published scientific staff offers expert technical support, proprietary protocols, and extensive know-how. For select projects, we develop the gene edited product specifically for our partner to commercialize, ensuring long term success. We invite researchers and industry to partner with us to develop the next breakthrough in biopesticides.
- Brizzee et al (2023) Fermentation.
- Madison et al (2022) Molecular Therapy Nucleic Acids.
- Brizzee et al (2023) Journal of Fungi.
- Stewart et al. (2023) Insects.
Validated In Mammalian Cells, Yeast, and Plants
We specialize in multiple industries, allowing us to apply Cas-CLOVER and piggyBac technologies into broad, real world applications.

Pharmaceutical Bioprocessing
Bioprocessing and cell line engineering to produce human or non-human therapeutics
Synthetic Biotechnology
Bioprocessing and strain improvement to produce therapeutics, industrial enzymes, compounds or biofuels
Agriculture Biotechnology
Enable plant modifications that may not require GMO labels and for the production of novel therapeutics
Optimizing Your Gene Editing Research?
Contact us to learn more about our innovative gene editing technology.