CleanCut GS CHO
Glutamine synthetase (GS) selection remains the industry benchmark for the rapid identification of high-titer suspension CHO cell clones in the production of recombinant therapeutic proteins. Demeetra's CleanCut GS knockout suspension cells are engineered using the proprietary Cas-CLOVER targeted nuclease technology. This system, unlike the monomeric editing platforms like CRISPR/Cas9, utilizes two separate guide RNAs and deactivated Cas9 (dCas9) solely for DNA binding, without cleavage. Instead, the dimeric Clo051 nuclease executes precise cleavage at the target loci, which enhances accuracy and markedly reduces unwanted off-target mutations and translocations (1,2).
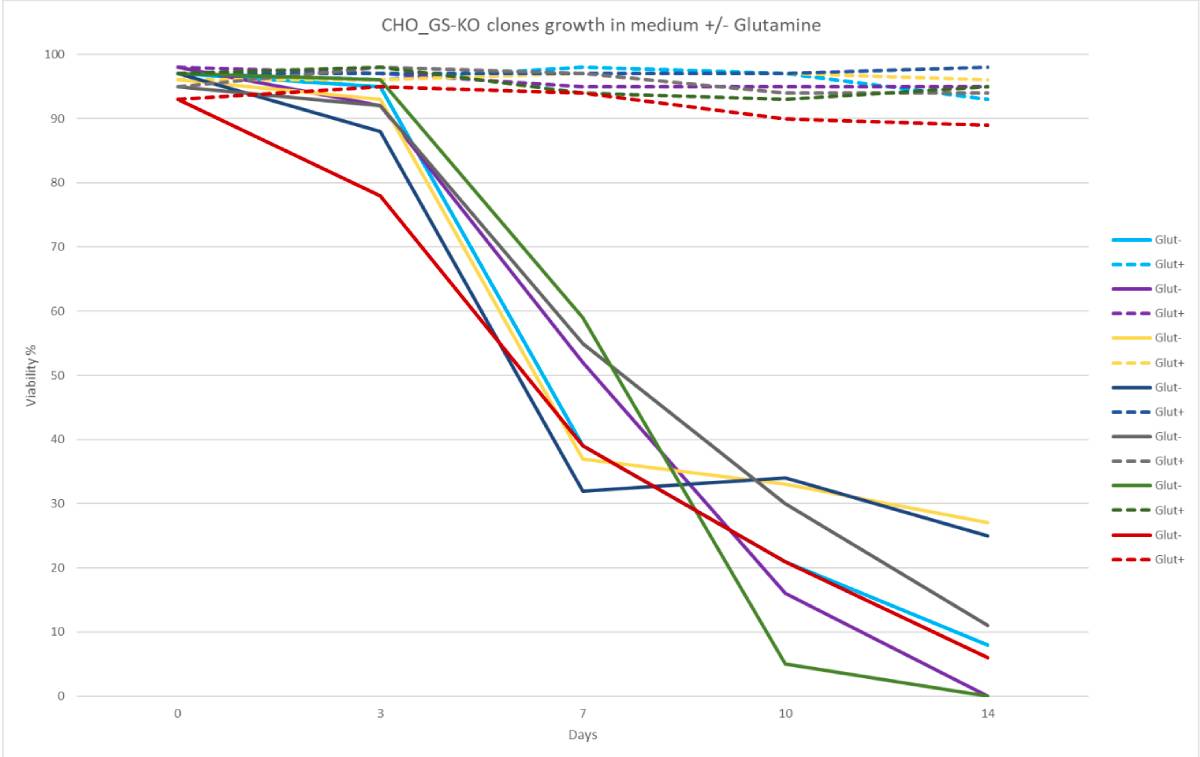
Furthermore, Cas-CLOVER technology facilitates the creation of larger deletions, potentially leading to more effective gene knockouts. The complete GS knockout was verified through glutamine sensitivity and viability assays. Multiple clones exhibited exceptional viability and recovery times, with doubling times under 20 hours (3).
Key Features
- GS knockout achieved using the ultra-precise Cas-CLOVER technology — a clean alternative to CRISPR/Cas9.
- Titers reached grams per liter at an unoptimized pilot scale, indicating significant potential for improvement.
- Demonstrated capability with challenging-to-express monoclonal antibodies (mAbs), such as Rituximab.
- Robust growth parameters include viability, recovery, and rapid doubling times.
- The cell line history package demonstrates manufacturing processes consistent with regulatory compliance, featuring automated single-cell clonal visual reporting.
- Flexible Evaluation and Commercial Licensing options available.
- Potential for integration with Demeetra IP and reagents for Cas-CLOVER or transposases for advanced cell line development.
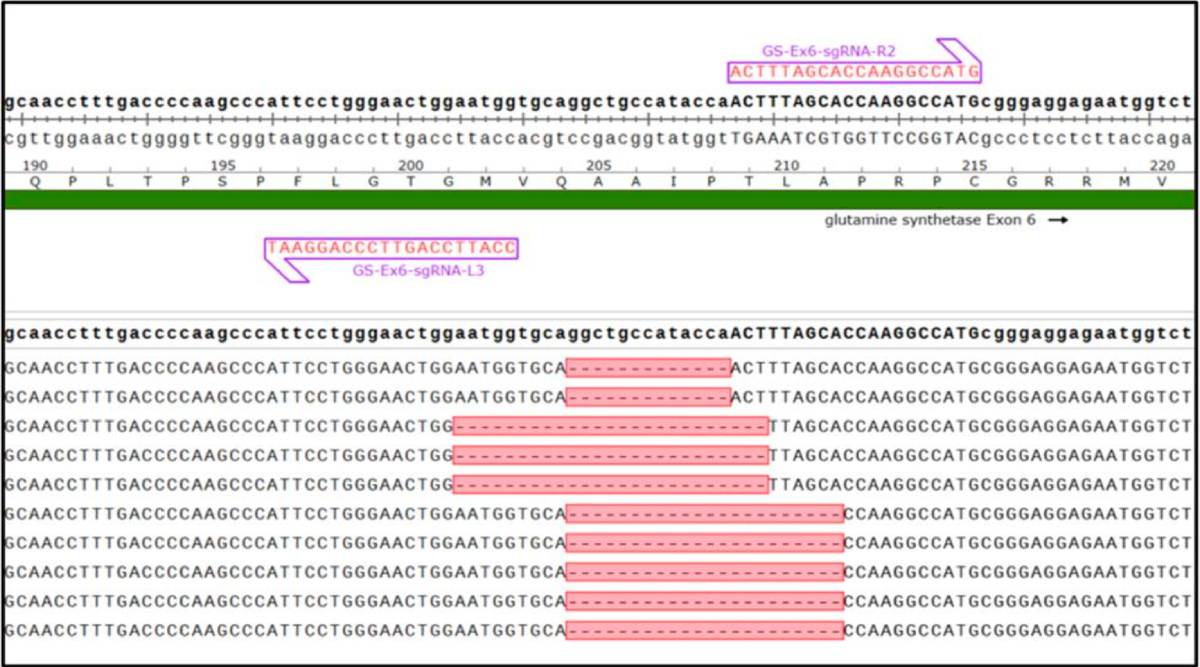
Guide RNA strategy and characterization of mutants. The top image illustrates left and right pair binding sites of gRNAs to the CHO GS gene. The bottom sequence alignments following Cas-CLOVER mediated targeting of GS in CHO individual clones. Substantial deletions and frameshift in exon 6; 13, 22 and 25 bp deletions are shown. Large deletions are routine to introduce using Cas-CLOVER.
Case Study: Rituximab
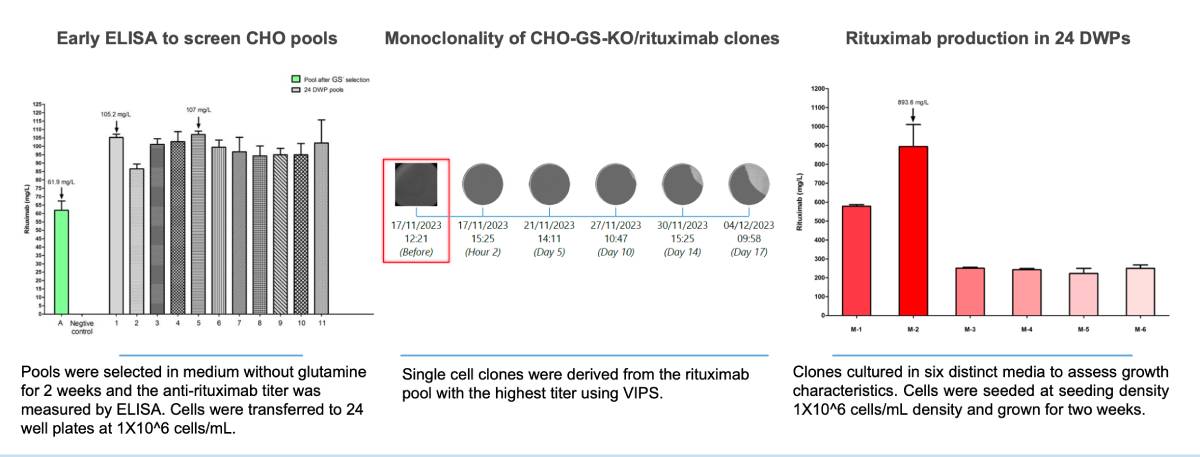
Rituximab, the first monoclonal antibody approved for therapeutic use, targets the CD20 antigen on B cells, effectively treating certain autoimmune diseases and cancers. Previously, process intensification strategies such as fed-batch, medium exchange, perfusion, and hybrid modes were necessary to significantly enhance Rituximab yields towards 1-2 g/L (4). By employing CleanCut GS CHO cells, Rituximab heavy and light chain genes were stably integrated, and preliminary clones achieved titers nearing 1000 mg/L. This successful demonstration underscores the potential of CleanCut GS CHO cells in streamlining cell line development and possibly reducing the need for extensive process intensification in upstream bioprocessing.
Licensing and Commercialization
Accessible licensing options that support commercialization efforts are available. For more information, please contact us.
Demeetra AgBio, Inc., holds the exclusive licenses for the gene editing technologies Cas-CLOVER and piggyBac® transposase, for use in research and commercial applications in agriculture, synthetic biotechnology, and pharmaceutical bioprocessing
Citations
- Madison (2022) Cas-CLOVER is a novel high-fidelity nuclease for safe and robust generation of TSCM-enriched allogeneic CAR-T cells. Mol Ther Nucleic Acids.
- Rozhdestvensky (2023) CRISPR/Cas9 Landscape: Current State and Future Perspectives. Int J Mol Sci.
- Steffey (2024) The CHOlympian Platform. Poster Presentation at Bioprocessing International West 2024.
- Mellahi (2019) Process intensification for the production of rituximab by an inducibleCHO cell line. Bioprocess and Biosystems Engineering.
Validated In Mammalian Cells, Yeast, and Plants
We specialize in multiple industries, allowing us to apply Cas-CLOVER and piggyBac technologies into broad real world applications.

Pharmaceutical Bioprocessing
Bioprocessing and cell line engineering to produce human or non-human therapeutics
Synthetic Biotechnology
Bioprocessing and strain improvement to produce therapeutics, industrial enzymes, compounds or biofuels
Agriculture Biotechnology
Enable plant modifications that may not require GMO labels and for the production of novel therapeutics
Optimizing Your Gene Editing Research?
Contact us to learn more about our innovative gene editing technology.