Key Features for Cell Line Development and Bioprocessing
- GS knockout achieved using the ultra-precise Cas-CLOVER technology — a clean alternative to CRISPR/Cas9.
- Industry-leading productivity with >70 pg/cell/day (Qp).
- Demonstrated capability with challenging-to-express monoclonal antibodies (mAbs), such as Rituximab.
- Robust growth parameters include viability, recovery, and rapid doubling times.
- The cell line history package demonstrates manufacturing processes consistent with regulatory compliance, featuring automated single-cell clonal visual reporting.
- Potential for integration with Demeetra gene editing IP and reagents for Cas-CLOVER or transposases for advanced cell line development.
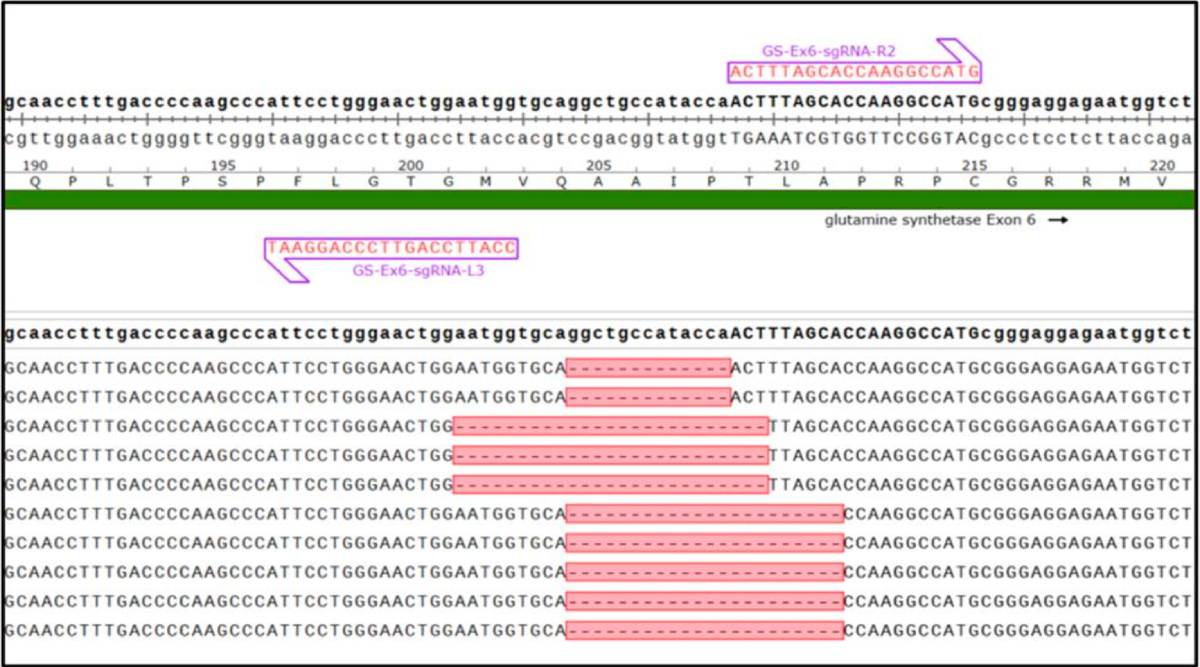
Guide RNA strategy and characterization of mutants. The top section of the image shows the binding sites of a guide RNA pair within the CHO GS gene. Below this, sequence alignments are presented following Cas-CLOVER mediated targeting of GS in individual CHO clones. Significant deletions and frameshifts in exon 6, including 13, 22, and 25 base pair deletions, are depicted. Large deletions are commonly introduced using Cas-CLOVER, resulting in complete knockout phenotypes.
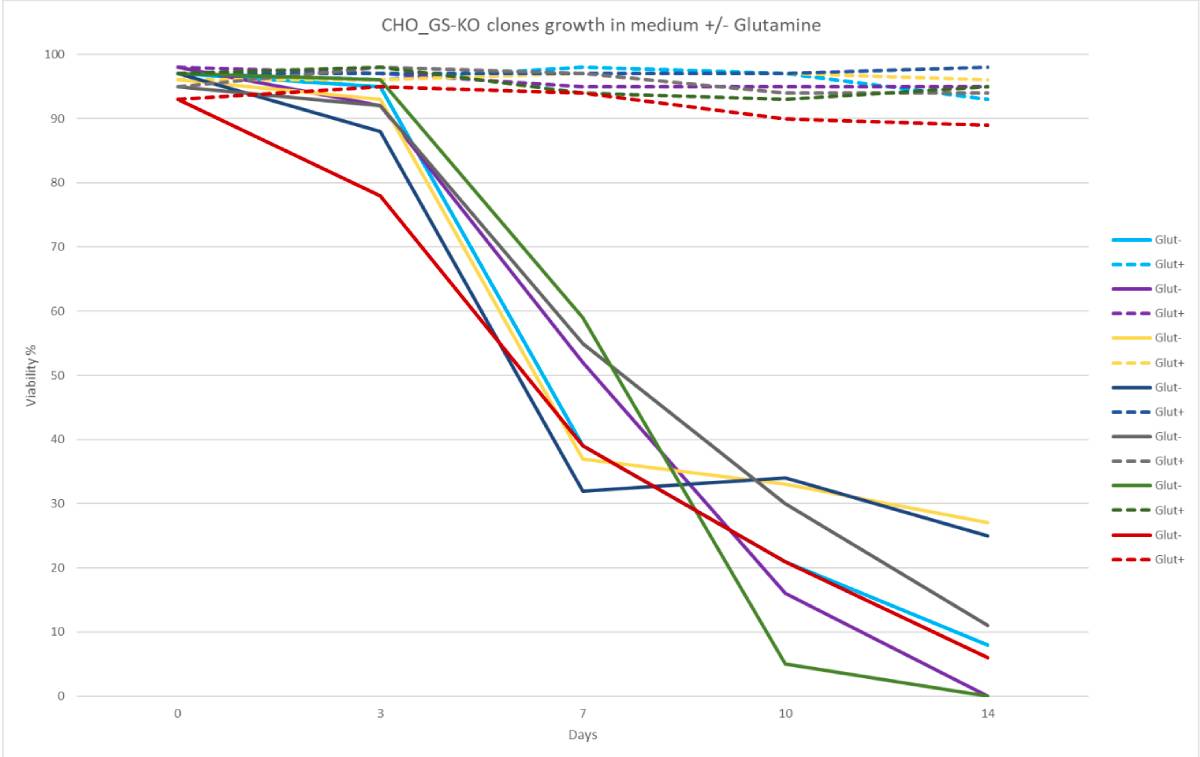
Sensitivity of CleanCut GS CHO clones to glutamine. Cell lines
show consistently decreased viability in medium without
glutamine, indicating a full knockout phenotype.
Monoclonality of CleanCut GS CHO clones. Single cell clones were derived using Solentim VIPS®.

Bioprocessing Case Study: Trastuzumab
Trastuzumab (Herceptin), a monoclonal antibody, is approved for the treatment of HER2-positive breast cancer. The heavy and light chain genes were stably integrated, with pools achieving an impressive >70 pg/cell/day (Qp). This successful demonstration underscores the potential of CleanCut GS CHO cells in streamlining cell line development.

