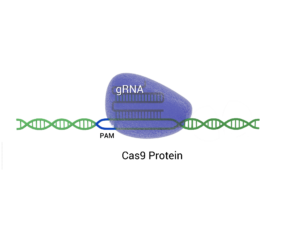
Emmanuelle Charpentier and Jennifer Doudna were awarded the 2020 Nobel Prize in Chemistry for discovering CRISPR/Cas9 ‘genetic scissors’ for editing DNA in an in vitro system, published in Science back in June 2012 (1). CRISPR/Cas9 is derived from a bacterial immune system in which guide RNAs carry the Cas9 protein to cut out specific regions of DNA.
It’s a powerful tool because earlier gene-editing methods, like zinc finger nucleases (ZFNs) and TALENs, demanded substantial protein engineering efforts. In contrast, CRISPR/Cas9 technology simplifies this process by utilizing a single, easily customizable and synthesizable guide RNA (gRNA). This gRNA steers the Cas9 enzyme to the desired DNA sequence, allowing it to attach and cleave the DNA at that point, which facilitates targeted genetic alterations.
Following its release, academic laboratories led the way in adopting CRISPR/Cas9 technology for use in cells, microbes, and plants. Additionally, there is considerable optimism that genome editing might one day serve to treat numerous genetic diseases via gene and cell therapy.
Freedom to Operate Issues Roadblock Cas9’s Progress
A prominent milestone in the use of CRISPR/Cas9 occurred when scientist Feng Zhang of the Broad Institute of MIT and Harvard validated the use of CRISPR/Cas9 in a mammalian model. Unfortunately, these dual CRISPR/Cas9 discoveries have been overshadowed by a large legal battle over the intellectual property rights. Scientists interested in commercial applications have encountered complicated licensing which has deterred adoption.
How Cas-CLOVER Improves On CRISPR/Cas9
The Cas9 system results in small, blunt-ended cuts and leverages cellular DNA repair mechanisms—Non-Homologous End Joining (NHEJ) or Homology-Directed Repair (HDR)—to introduce deletions or insertions (Indels) at the cleavage site. Since Cas9 is monomeric and a single guide RNA is used, CRISPR/Cas9 has been reported to have significant off-target mutations and creates genomic translocations.
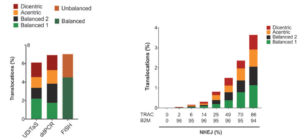
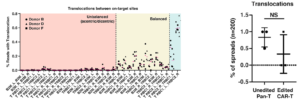
CRISPR/Cas9 blunt-ended cleavage induces on-target and off-target translocations which can cause genomic instability. In primary human T-cells, three different detection methods were employed to characterize the overall level and types of chromosome rearrangements: 1- Uni-Directional Targeted Sequencing (UDiTaS). 2- droplet digital PCR (ddPCR). 3- fluorescent in situ hybridization (FISH). As shown CRISPR/Cas9 cleavage results in a consistently high 7% translocation rate (left panel). The translocation percentage increases exponentially with NHEJ percentage, making for a difficult efficiency/precision balance when using Cas9 (2) (right panel). Recently, even the most advanced versions of Cas9 technology, base and prime editing, have been shown to produce more off-target mutations than previously thought (3).
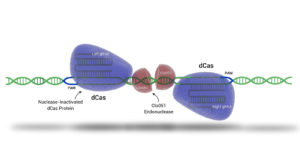
Cas-CLOVER is a gene editing system that shares many benefits with CRISPR/Cas9 but has distinct advantages due to its unique components. Unlike CRISPR/Cas9, which uses a single guide RNA (gRNA) and the Cas9 enzyme to cut DNA, Cas-CLOVER employs two separate gRNAs and a deactivated Cas9 (dCas9) for DNA binding without cutting. Instead of Cas9, the cutting action is performed by the dimeric nuclease Clo051, enhancing the system's precision and significantly reducing unwanted and off-target mutations. Additionally, Cas-CLOVER creates larger 4-base pair overhang deletions, which can lead to more complete gene knockouts and improved efficiency for gene insertions (knock-ins). The distinctive mechanics of Cas-CLOVER not only improve its editing fidelity but also establishes its own intellectual property space, providing freedom to operate independently from CRISPR/Cas9 patents.
In contrast to CRISPR/Cas9, anchored multiplex PCR and NGS (AMP-seq) reveals Cas-CLOVER’s translocation rates to be minimally 10-fold lower from 0 to 0.7% (left panel). Further, karyotype analysis also showed no differences in translocations between edited and unedited T-cells (right panel). Unlike Cas9, Cas-CLOVER maintains genomic stability at very high on-target NHEJ percentage (92-99% in these data), eliminating the risks with high efficiency gene editing in cells (4).
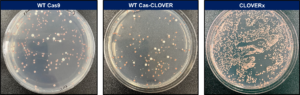
In the yeast, Saccharomyces cerevisiae (S. cerevisiae), deleting the ade2 gene exhibits a red colony color phenotype through intermediate metabolite accumulation of the adenine biosynthesis pathway. We used this simple colony count assay as a method for testing gene editing efficiencies of CRISPR/Cas9 vs. Cas-CLOVER.
The ade2 assay provides a visual comparison between gene editing technologies. CRISPR/Cas9 (left panel) and Cas-CLOVER (middle panel) have similar performance, while our newest version CLOVERx (right panel) has a striking increase in efficiency and number of colonies.
While the world attests that CRISPR/Cas9 has truly revolutionized the world of genetics, it’s time to consider that the latest technology—like Demeetra's Cas-CLOVER—could be even more useful.
Learn more about Cas-CLOVER and our Licensing Options.
References:
1 Jinek (2012) Science
2 Bothmer (2020) CRISPR J.
3 Fiumara (2023) Nat. Biotech.
4 Madison (2022) Molecular Therapy - Nucleic Acids