In contemporary society, the practice of shopping on a weekly rather than daily basis presents challenges in maintaining the freshness of produce. Preserving produce is critical for economic efficiency and mitigating greenhouse gas emissions resulting from food waste.
Advanced gene editing technology facilitates precise targeted mutations without the introduction of transgenes, enabling the deactivation of specific plant genes to achieve desirable traits. One such trait is the extended shelf life of produce. The tomato represents an ideal candidate for this technology, as data from the USDA indicates that spoiled tomatoes result in over $4 billion in economic losses annually in the United States (1).
Food waste significantly contributes to methane emissions, which have a higher global warming potential than carbon dioxide. According to the EPA, in the U.S., food waste constitutes 24% of landfill municipal solid waste and produces 58% of landfill methane emissions (2). Approximately 31% of fresh tomatoes are discarded by households, and are therefore a significant methane producer.
Why Target Pectin for Gene Editing?
Pectin's integral role in the texture of tomatoes has made it a key focus for gene editing aimed at enhancing fruit firmness. A recent study utilized advanced DNA editing technology, specifically CRISPR/Cas9, to generate loss-of-function mutants to investigate the roles of specific cell wall structural enzymes.
Researchers examined the impact on fruit softening and pectin localization within the pericarp (cell wall) of ripe tomatoes by introducing mutations in genes encoding pectin-degrading enzymes: pectate lyase (PL), polygalacturonase 2a (PG2a), and β-galactanase (TBG4).
The study highlighted the efficacy of gene editing technologies, demonstrating that these three enzymes are crucial for typical ripening-related modifications in cell-to-cell adhesion. The loss of function resulted in diminished ripening-associated activities.
Ripening-related characteristics were evaluated through various metrics. Each pectin-degrading enzyme exhibited distinct functions: mutations in PL led to firmer fruits, while PG2a and TBG4 mutations influenced color and weight. The research also assessed pectin localization, distribution, and solubility in the fruit cell wall. Elevated levels of de-esterified homogalacturonan in the cell walls of the PL, PG2a, and TBG4 mutant lines compared to the control indicated reduced pectin degradation in the knockout lines (3).
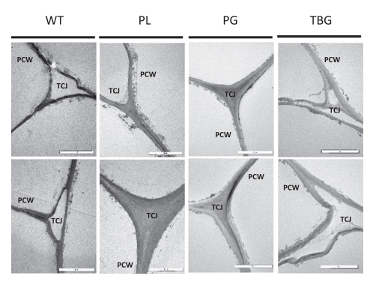
Sections cut from wild-type and the three mutated PL5, PG34, and TBG-8 lines were visualized under the transmission electron microscope and two representative micrographs shown for each line. The mutated fruits often appear larger than in other lines.
New Gene Editing Technologies Like CRISPR And Cas-CLOVER
Novel gene editing proteins, such as Cas-CLOVER, Cas9, transcription activator-like effector nucleases (TALENs), and zinc-finger nucleases (ZFNs), enable precise targeting of specific genes. This is in contrast to earlier methodologies that inserted, deleted, or caused frameshifts randomly within the sequence being edited, resulting in significant alterations to gene function.
Previous research on fruit ripening enzymes utilized gene technologies like RNA interference (RNAi), which reduces gene expression at the mRNA level. Contemporary technologies, such as CRISPR/Cas9 and Cas-CLOVER, however, permanently silence genes at the DNA level without incorporating exogenous transgenes into the plant. This method offers a permanent solution compared to the transient effects of RNAi but also addresses the limitations of RNAi’s high off-target effects and the transgenic GMO status associated with RNAi methods.
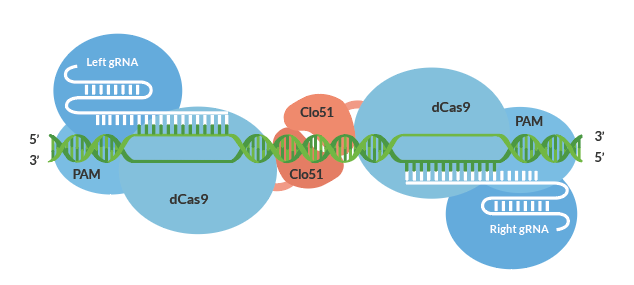
Cas-CLOVER was initially introduced and validated to exhibit minimal off-target effects due to its dimeric nature in cell lines, in human T-cells (4). It employs a catalytically inactive Cas9 protein fused to the Clo51 nuclease domain, which functions as monomers recruited by a pair of guide RNAs (gRNAs) to introduce targeted mutations. Upon correct recruitment of both subunits to the target site, dimerization and activation of the Clo51 nuclease domain occur, resulting in precise gene disruptions.
Demeetra’s Advanced Gene Editing System Validated For The Plant Genome
Advanced gene editing technologies enable plant breeders with the ability to make targeted knockouts. This scarless gene editing means no heterologous genes are left in the plant genome, producing a non-GMO crop and simplifying the commercialization process. Shown in a previous post, we validated the activity of Cas-CLOVER in plants by targeted inactivation of the phytoene desaturase (PDS) gene in tobacco achieving an efficiency of 90%. Enhancing tomato production is just one example that can benefit from Cas-CLOVER, Schedule A Call to learn more.
References
- Buzby et al (2011) The Value of Retail- and Consumer-Level Fruit and Vegetable Losses in the United States. Journal of Consumer Affairs. https://doi.org/10.1111/j.1745-6606.2011.01214.x
- EPA Website. Quantifying Methane Emissions from Landfilled Food Waste.
- Wang, D., Samsulrizal, N. H., Yan, C., Allcock, N. S., Craigon, J., Blanco-Ulate, B., … Seymour, G. B. (2019). Characterization of CRISPR mutants targeting genes modulating pectin degradation in ripening tomato 1[OPEN]. Plant Physiology, 179(2), 544–557. https://doi.org/10.1104/pp.18.01187
- Madison et al (2022) Cas-CLOVER is a novel high-fidelity nuclease for safe and robust generation of TSCM-enriched allogeneic CAR-T cells. Molecular Therapy Nucleic Acids.