Cas-CLOVER significantly reduces off-target risk with a paired gRNA, deactivated Cas fusion protein (dCas), and dimeric Clo051 nuclease. A proven technology for cell line development (CLD) and bioprocessing, agriculture, and synthetic biotechnology, Cas-CLOVER also features high efficiency in target cells and organisms coupled with simple and flexible design and technology licensing (Figure 1).
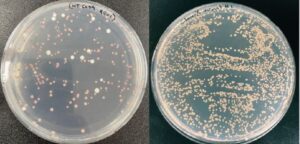
Figure 1. Plates containing gene edited yeast: left: Cas9; right: CLOVERx. Correctly targeted gene editing produced red colonies.
“One common question is, ‘Without Cas9, how do we design the guides?’” says David Norman, senior scientist at Demeetra. “Some have struggled with other alternatives to Cas9 where less is known about guide design. Cas-CLOVER seamlessly works with your favorite design platforms and gRNA manufacturers.”
Cas-CLOVER’s flexible spacer between the two gRNAs required for Clo051dimerization enables target accessibility. “In cells, yeast and plants, high activity between 12 and 32 base spacers is common. I’ve never seen a sequence we couldn’t target. Our guidance is to start with spacers of 15–30 bases,” Norman says. (See Figure 2 and Figure 3).
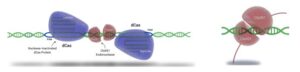
Figure 2. Cas-CLOVER utilizes two gRNAs and requires dimerization of the subunits associated with each gRNA.
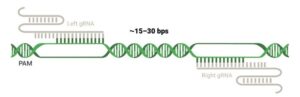
Figure 3. Cas-CLOVER is strictly controlled by on-site target binding of both gRNAs within a specific spacer length.
“The end result is beneficially distinct from CRISPR-Cas9, because it’s a completely different nuclease. While Cas9 typically results in smaller blunt-end deletions, Clo051 yields larger deletions with four-base overhangs,” Norman continues. Large deletions are easier to identify, cutting down workflow processes. Researchers have also observed higher knock-in efficiency with Cas-CLOVER using homology-directed repair (HDR) and non-homologous end joining (NHEJ).
Maintaining the ROI for geneediting technology
Gene editing can improve biotherapeutics; the manufacturing of viral vectors; cultured meats and proteins; and even agriculture. However, economic barriers and FTO confusion have slowed commercial use. “We want Cas-CLOVER applied for maximum ROI for our partners,” says Jack Crawford, CEO at Demeetra. “For established companies with large pipelines, an ideal license structure is much different than for CDMOs working on a cash-flow basis or start-ups looking to get to the next value inflection.” Demeetra has established a flexible license framework for all situations. “If Cas-CLOVER can help, we want to do the deal now and on mutually beneficial terms for licensees and, ultimately, society,” Crawford adds.
A beneficial agricultural revolution
Cas-CLOVER in plants is a growing success in both dicots and monocots. One such project is with Leena Tripathi at the International Institute of Tropical Agriculture (IITA), Kenya. Tripathi group and Demeetra collaborated to test Cas-CLOVER in banana plants. Delivering the Cas-CLOVER reagents to banana cell suspension and targeting the PDS gene resulted in the PDS knockout. This revealed the expected albino phenotype, and sequencing later confirmed large deletions associated with Cas-CLOVER (Figure 4).

Figure 4. Albino phenotype with Cas-CLOVER-transformed banana.
“We are very excited to improve the banana varieties preferred by farmers in Africa for disease-resistant bananas using Cas-CLOVER”, Tripathi says. “The gene edited banana with no foreign gene integration will not be regulated as a GMO in Kenya. The disease-resistant varieties will enhance banana production and increase the incomes of smallholder farmers in East Africa, where the banana is one of the major staple foods.”
Engineering for disease resistance and delayed browning are next on the list. Traits for varieties that are sold locally in Africa will not be royalty bearing, while varieties that are exported such as Cavendish will be licensed by Demeetra. “We have set up a robust process with Leena’s group and are open to partnering with banana export companies, while providing unhindered value to African food security.” Crawford says.
Unlike most traditional agricultural companies, Demeetra does not plan on providing seed or managing acreage. Instead, Cas-CLOVER is licensed for research that can convert to flexible commercial access. “Clients can edit their target crop in-house or can use any CRO, including Demeetra, or academic group to create the edits they desire,” Crawford says. “Since we do not ourselves have an interest in any crop, other than for manufacturing cannabinoid drug candidates, we can be very creative with our license structure—and the client owns all the IP created with the technology.”
Can it get any better?
“Imagine nearly 100% targeted editing with under 1% off-target risk. That is precisely what we are aiming for with our new Clo051 variants,” says Corey Brizzee, strain engineering scientist at Demeetra. Through robust yeast gene editing and protein engineering capabilities, Demeetra has discovered hyperactive Clo051 mutants that reach 99% targeted editing as well as 10 times less cellular toxicity compared to Cas9 in yeast (Figure 1).
New hyperactive variants which Demeetra refers to as “CLOVERx” are expected to apply to all projects but will be especially important for organisms that require more resources such as plants and agricultural animals. “Our licenses have always included future technology improvements; this is another benefit of partnering with Demeetra,” Crawford concludes. These improvements to the platform are similar to updates to operating software—improving performance on a complimentary basis.
