Biopharmaceuticals, encompassing monoclonal antibodies (mAbs) and their derivatives such as Fc-fusion proteins, antibody-drug conjugates (ADCs), immunocytokines, and antibody-enzyme fusions, have introduced a novel paradigm in therapeutic modalities. This innovative category of therapeutics has made significant contributions to the treatment of malignancies, autoimmune diseases, and enzyme replacement therapies.
Similar to endogenous proteins, such as immunoglobulin G (IgGs), mAbs undergo glycosylation during their biosynthetic process. The glycans attached to IgG consist of complex biantennary structures. The stochastic nature of heavy-chain glycan pairing results in the potential formation of several hundred glycoforms, thereby introducing substantial heterogeneity1. This variability underscores the importance of glycosylation as a pivotal biochemical process that profoundly affects the structural and functional attributes of monoclonal antibodies, necessitating rigorous characterization over this modification to ensure therapeutic efficacy and safety.
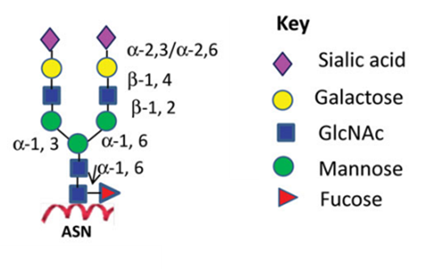
Figure 1:N-linked glycoforms of mAb therapeutics
Glycan heterogeneity and modifications can result in conformational changes to the biologic with profound effects on the function of the large molecule. Negative impacts to pharmacokinetics (PK) can occur - along with immune effector functions: complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cell-mediated phagocytosis (ADCP).
Improving The ADCC Of mABs
ADCC, a lytic attack on antibody-targeted cells, is solely controlled by the absence of fucose on the glycoforms (shown as the red triangle in Figure 1). It was established that the α-1,6-fucosyltransferase (FUT8) gene encodes the enzyme responsible for fucosylation of mAB glycoforms. In a 2004 publication a FUT8 knockout CHO cell line developed through sequential homologous recombination (HR), a difficult method with very low efficiency. The group demonstrated that FUT8-/-were defucosylated and that ADCC activity was enhanced in comparison to Rituxan which is made in a FUT8+/+ CHO cells2.
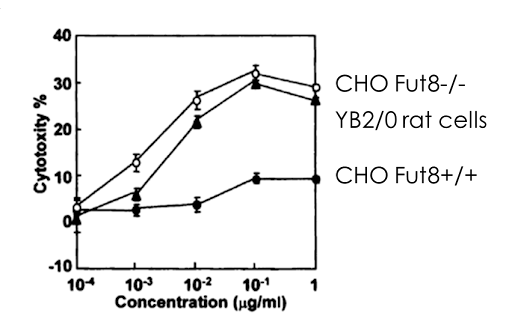
Figure 2: Enhanced ADCC of FUT8-/- CHO cells versus parental cell line. The ADCC is higher than a lesser utilized rat hybridoma cell line YB2/0 which is known to produce defucosylated antibodies.
This innovative FUT8-/- CHO cell proved its worth when a defucosylated mAB (KW-0761) targeting the CCR4 expressed on adult T-cell leukemia/lymphoma (ATLL) demonstrated enhanced efficacy in both mouse and human.
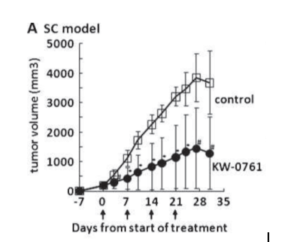
Figure 3: anti-tumor activity of defucosylated mAB KW-0761 produced in a FUT8 knockout CHO cell in an established mouse tumor model.
A Faster And Easier Path To The Ideal Cell Line
Although the original FUT8-/- CHO cell line was published in 2004; the employment of sequential homologous recombination (HR) as a methodology presented limitations and may pose challenges for reproducibility. The efficiency of HR in CHO cells is notably low, more effective and readily available techniques, such as gene editing, exist as alternatives. Although the CRISPR/Cas9 system is effective, its potential to induce off-target mutations, translocations and its complex freedom to operate and licensing are significant concerns for commercial bioprocessing.
Demeetra's Cas-CLOVER system, has demonstrated highly efficient knockouts and knock-ins in CHO & HEK293 cells and has been used to target glycosylation related genes for biotherapeutic enhancement.
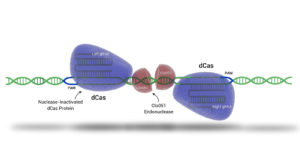
Cas-CLOVER is a gene editing system that shares many benefits with CRISPR/Cas9 but has distinct advantages due to its unique components. Unlike CRISPR/Cas9, which uses a single guide RNA (gRNA) and the Cas9 enzyme to cut DNA, Cas-CLOVER employs two separate gRNAs and a deactivated Cas9 (dCas9) for DNA binding without cutting. Instead of Cas9, the cutting action is performed by the dimeric nuclease Clo051, enhancing the system's precision and significantly reducing unwanted and off-target mutations
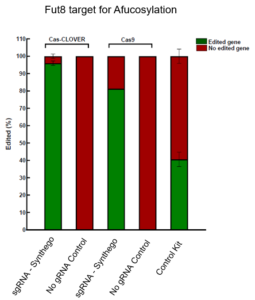
Cas-CLOVER demonstrates superior editing efficiency compared to CRISPR/Cas9 at the Fut8 gene in CHO cells, as evidenced by T7 endonuclease assay results.
With specificity that far exceeds that of CRISPR/Cas9, Cas-CLOVER may be ideal for targeted glycoengineering. Demeetra offers unparalleled technical support and proficiency in gene editing, providing more than mere access to tools and intellectual property licenses. Our collaboration encompasses exclusive protocols, expert knowledge, and direct technical assistance from our staff, whose excellence is validated by peer-reviewed publications. We invite you to contact Demeetra to explore how our gene editing solutions can be customized to address your unique research and development requirements.
References
- Liu et al. (2015) Antibody Glycosylation and Its Impact on the Pharmacokinetics and Pharmacodynamics of Monoclonal Antibodies and Fc-Fusion Proteins. J. Pharmaceutical Sciences.
- Yamane-Ohnuki et al. (2004) Establishment of FUT8 Knockout Chinese Hamster Ovary Cells: An Ideal Host Cell Line for Producing Completely Defucosylated Antibodies With Enhanced Antibody-Dependent Cellular Cytotoxicity.
- Ishii et al. (2010) Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clinical Cancer Res.
