Recombinant adeno-associated virus (rAAV) vectors are a prime example of how nature can be harnessed for biotechnology purposes, such as gene therapy. Viruses, including AAV, have evolved sophisticated mechanisms to enter host cells and deliver their genetic material. This natural proficiency for gene transfer makes them effective tools for introducing therapeutic genes into human cells. rAAV vectors are engineered to eliminate the virus' ability to replicate within a host cell and therefore can enter cells without producing new virus particles, thus minimizing the risk of triggering adverse immune responses or causing disease.
Roadblocks In Viral Vector Biomanufacturing
Despite the advancements in rAAV vectors; the path to developing new gene therapies and vaccine treatments is riddled with roadblocks. One key bottleneck is the development of scalable cell line manufacturing focused on high titer, purity and potency of viral vectors. From low-yielding biomanufacturing processes and low cell line engineering efficiencies platforms, it remains difficult to produce large quantities of virus. But this capability is crucial for clinical studies and commercially-produced gene therapies.
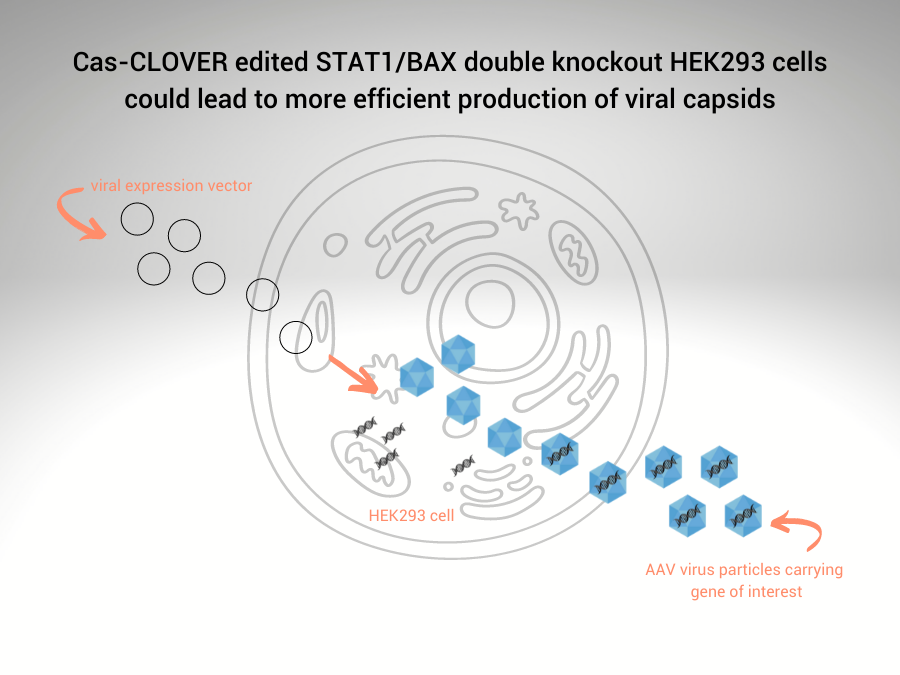
Recombinant adeno-associated virus (rAAV) expression vector(s) are transfected into suspension HEK293 cells, which produces viral capsids for delivery of the gene of interest. Purified viral particles can’t replicate but efficiently infect cells delivering therapeutic genes.
Gene Editing - Key Improvements To Virus Bioprocessing
HEK293 cells are a platform for simple and efficient transfection but not as widely used for large scale bioprocessing like its biologics producing counterpart Chinese Hamster Ovary (CHO) cells. In the first step, adherent HEK293 cells were adopted to animal component free suspension culture in shake flasks and WAVE bioreactors. Following several optimization steps scientists developed a 1-week process with significantly improved post-purification yields of full particles for multiple serotypes and chimeric capsids1.
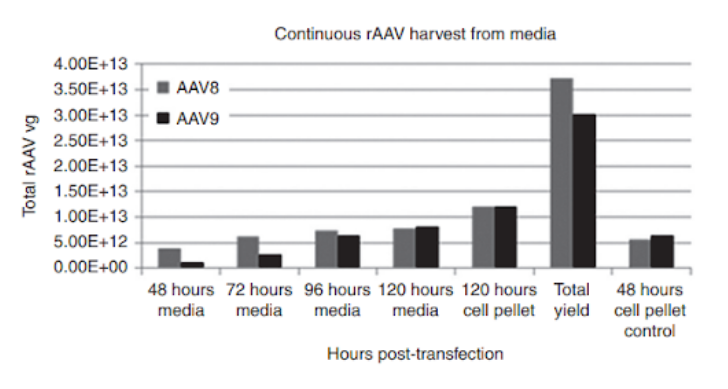
rAAV serotype AAV8 and AAV9 yield under the Grieger et al. continuous production process utilizing suspension HEK293 cells
While the majority of rAAV manufacturing still relies on traditional platforms such as transient transfection, more sophisticated cell line development improvements similar to those widely used in suspension CHO cells are starting to be implemented. Gene-editing technologies, such as CRISPR/Cas9 have recently been used by The American Type Culture Collection (ATCC) to improve manufacturing yields. Genes that produce proteins STAT1 and BAX are known to inhibit viral production in cells by producing interferon and other antiviral responses2.
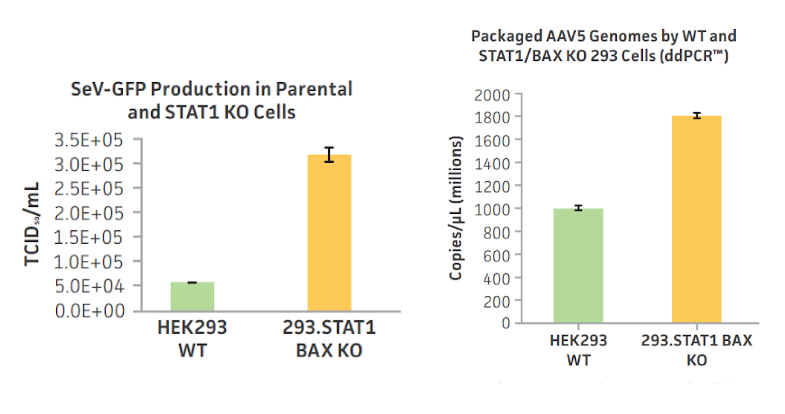
STAT1/BAX double knockout HEK293 cells demonstrate improved yields of Sendai virus and rAAV over their respective parental cell lines
But there are significant problems when editing cells with CRISPR/Cas9 such as its tendency to produce off-target mutations. The inaccuracy of CRISPR poses further issues downstream and can compromise the utility of cell lines. Additional disadvantages of these cells are that they are not the end-users proprietary cell lines and high upfront costs and royalty fees may be associated with Cas9's complex patent licensing landscape.
Demeetra's Cas-CLOVER Could Provide A Solution
Fortunately, a verified alternative to Cas9 is now available. In suspension HEK293 cell lines at genomic loci that are known to be safe-harbor sites, we found Cas-CLOVER’s activity significantly outperformed Cas9 as measured by Indel frequency in the T7 EnGen cleavage assay.
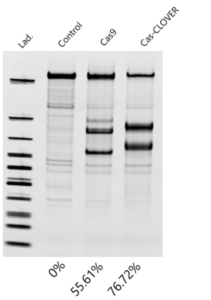
Indel frequency measured by T7 cleavage assay in HEK293 cells transfected with Cas-CLOVER and Cas9 (right gRNA) mRNA at the same target.
The Cas-CLOVER system, characterized by its novel integration of dual gRNA and Clo051 nuclease, facilitates the precise engineering of targets in HEK293 cells. This advancement represents a significant evolution in gene-editing capabilities, and establishing a distinct separation from the foundational CRISPR/Cas9 intellectual property landscape, thereby enhancing operational freedom.
We invite the scientific community to investigate the capabilities of the Cas-CLOVER technology. Leveraging Demeetra's superior gene editing expertise and comprehensive technical support, researchers gain access not merely to cutting-edge tools and IP licensing but to a collaborative partnership. This includes exclusive protocols, specialized knowledge, and direct support from our team of experts with a track record of peer-reviewed publications. We encourage inquiries to Demeetra for personalized consultations on how our gene editing solutions can be adapted to fulfill the specific objectives of your research and development projects.
References
- Grieger (2016) Production of Recombinant Adeno-associated Virus Vectors Using Suspension HEK293 Cells and Continuous Harvest of Vector From the Culture Media for GMP FIX and FLT1 Clinical Vector. Molecular Therapy
- ATCC website. https://www.atcc.org/resources/application-notes/generation-of-cell-lines-capable-of-producing-high-titer-viral-stocks
