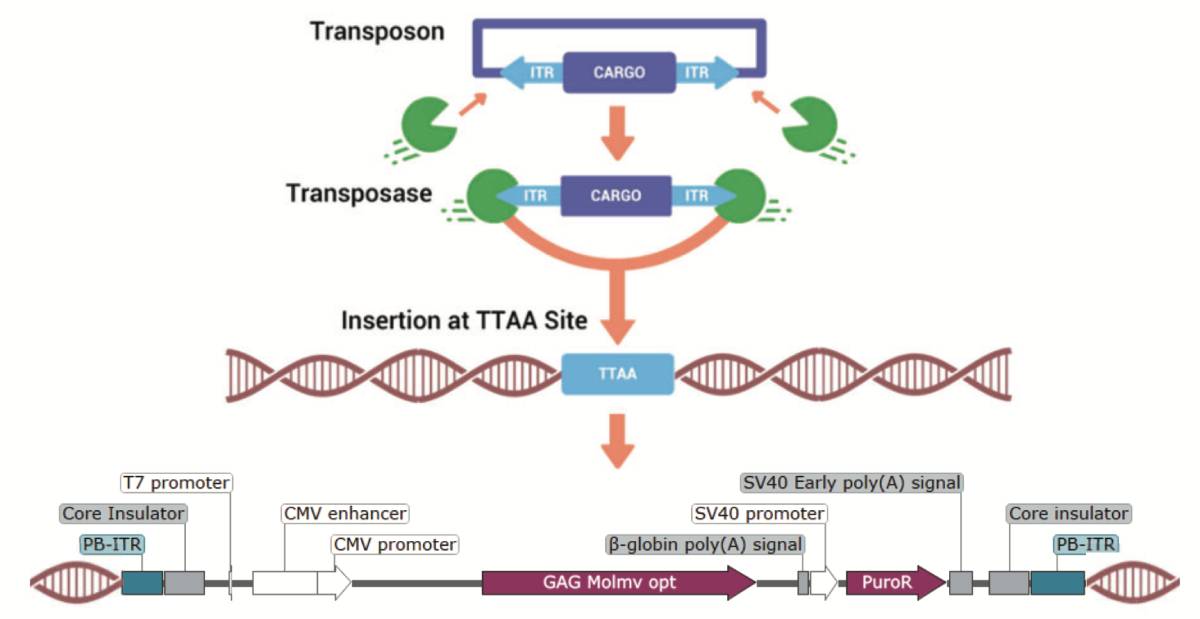
Poster Presentation: piggyBac® Transposase and Cas-CLOVER™ Targeted Nuclease Enable Rapid Discovery of Novel Hot Spots in HEK293 Suspension Cells
Poster Presentation: piggyBac® Transposase and Cas-CLOVER™ Targeted Nuclease Enable Rapid Discovery of Novel Hot Spots in HEK293 Suspension Cells Cell factory platforms are used in commercial bioproduction to express therapeutic molecules such as antibodies, vaccines, and biosimilars. Through innovative gene editing tools, cell lines can be tailor-made to stably express proteins at varying levels. The…
Read MoreDemeetra Cas-CLOVER Research Selected as a MDPI Journal Editor’s Choice Article
Demeetra Cas-CLOVER Research Selected as a MDPI Journal Editor’s Choice Article Lexington, KY and Bloomington, IN: A pioneering study, authored by scientists from Demeetra and Indiana University, has been selected for the MDPI Journals Editor’s Choice Articles, highlighting the impact of advanced gene editing in synthetic biology to produce biopesticides. Published in Fermentation in 2023, Generation…
Read MoreGEN Webinar: Cas-CLOVER: The Clean Alternative to CRISPR-Cas9
In this 2020 introductory webinar “Cas-CLOVER: A Clean Alternative to CRISPR,” two experts present data on Cas-CLOVER fundamentals such as architecture, design and implementation in cells, yeast, plants as well as advanced gene editing in human T-cells for allogeneic CAR-T therapies. Thanks to GEN: Genetic Engineering and Biotechnology News for hosting.
Read MoreDemeetra’s Guide to CRISPR and Gene Editing Licensing
Demeetra’s Guide to CRISPR and Gene Editing Licensing Figure 1: A general schematic of CRISPR’s simple single gRNA mechanism and function. Includes two outcomes: error-prone repair, which generally results in a LOF mutation, and template-based repair where genetic functionality is altered and genome is changed. While today many scientific articles present a simple process of…
Read MoreERS Genomics & Demeetra Enter Into CRISPR/Cas9 Licensing Agreement
Dublin, Ireland and Kentucky, USA, September 12: ERS Genomics Limited (‘ERS’) is pleased to announce a new license agreement with Demeetra AgBio (‘Demeetra’). This is a non-exclusive licensing agreement granting Demeetra access to the ERS CRISPR/Cas9 patent portfolio which is sublicensable when combined with Cas-CLOVER.
Read MoreGuide RNA Design For Cas-CLOVER
Dimerization of our Cas-CLOVER gene editing technology requires the design of two guide RNAs (gRNAs) to the gene of interest.
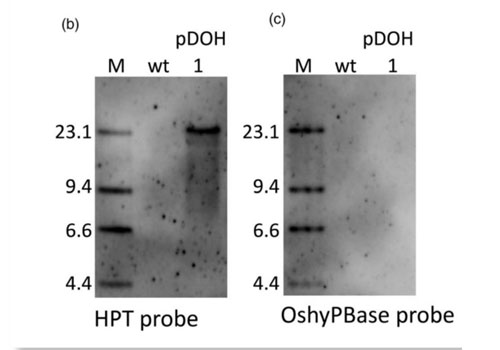
Read MoreA piggyBac-mediated transgenesis system for the temporary expression of CRISPR/Cas9 in rice
Targeted mutagenesis via CRISPR/Cas9 is now widely used, not only in model plants but also in agriculturally important crops. However, in vegetative crop propagation, CRISPR/Cas9 expression cassettes cannot be segregated out in the resulting progenies, but must nevertheless be eliminated without leaving unnecessary sequences in the genome. To this end, we designed a piggyBac-mediated transgenesis…
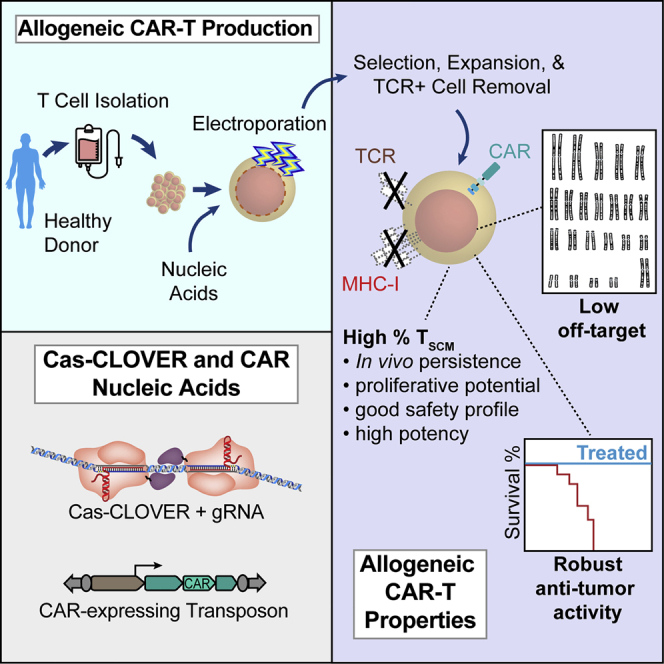
Read MoreCas-CLOVER is a novel high-fidelity nuclease for safe and robust generation of TSCM-enriched allogeneic CAR-T cells
Abstract The use of T cells from healthy donors for allogeneic chimeric antigen receptor T (CAR-T) cell cancer therapy is attractive because healthy donor T cells can produce versatile off-the-shelf CAR-T treatments. To maximize safety and durability of allogeneic products, the endogenous T cell receptor and major histocompatibility complex class I molecules are often removed…
Read MoreThe Ultimate Guide to Gene Editing in Wheat
The Complicated History of Wheat Wheat has had a complicated history of dispersion, adaptation, and selection since it was domesticated in the Fertile Crescent between 8,000 and 10,000 years ago. As humans migrated from the African forests into savannah regions around 6 million years ago, grass species with small, hard seeds quickly entered the human…
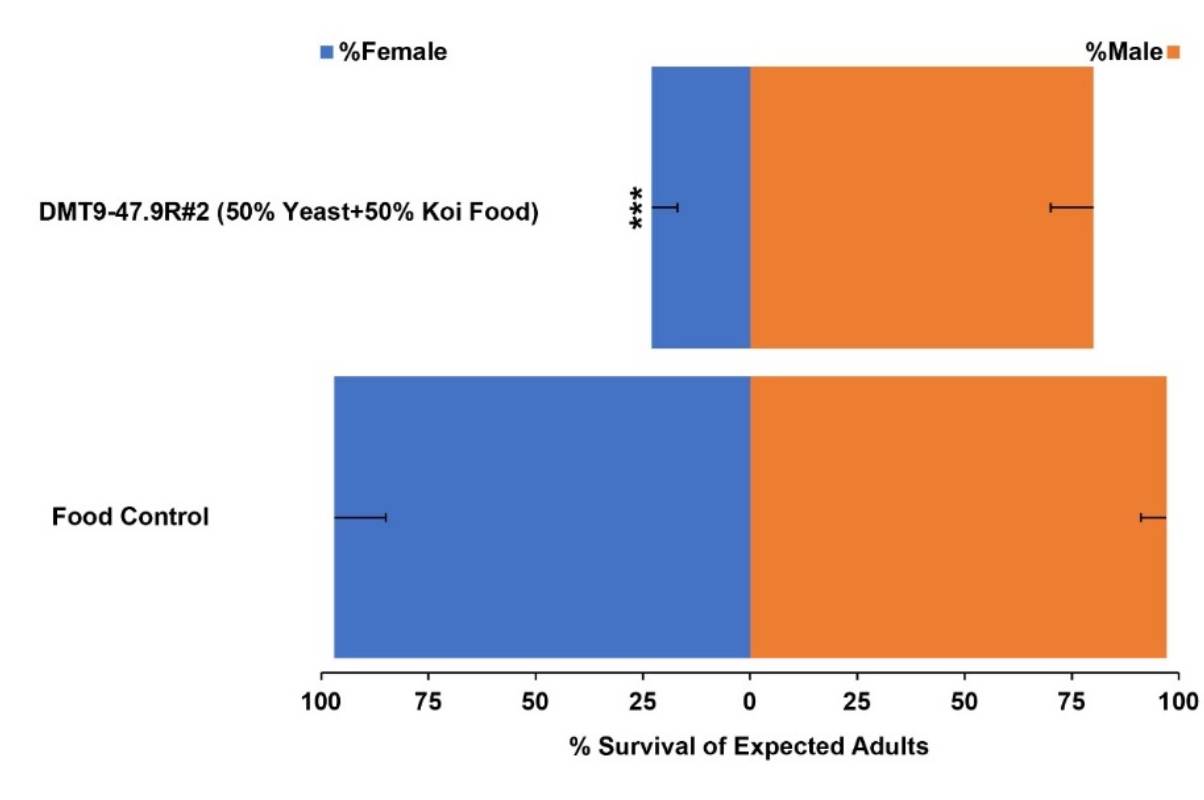
Read More89th American Mosquito Control Association Annual Meeting
Meet with the Demeetra team at Booth #511 to chat about the latest development strategies in mosquito control and to gain an intimate understanding of our cutting-edge larvicidal research.
Read More